INTRODUCTION
COVID-19 is associated with coagulopathy that correlates with poor prognosis. Although the underlying mechanism of COVID-19 coagulopathy remains unknown, early reports suggested that it may be a form of disseminated intravascular coagulation (DIC). However, recent studies have highlighted the potential role of endothelial cell injury in its pathogenesis.
AIMS
The aims of our study were to analyze the coagulation parameters of critically and non-critically ill patients with COVID-19 pneumonia admitted to our hospital, determine if coagulation factors consumption occurs, identify potential prognostic biomarkers of this new disease and explore possible underlying mechanisms of COVID-19 coagulopathy.
METHODS
We conducted a retrospective cohort study performed at Gregorio Marañon Hospital in Madrid, Spain. Adult patients with a diagnosis of COVID-19 hospitalized in our center were recruited, including those admitted to the ICU and to general wards. Patients were randomly selected from blood samples that arrived at our Hemostasis laboratory during April 2020. For each patient, we conducted a complete analysis of coagulation parameters, including basic coagulation tests, quantification of coagulation factors and physiological inhibitor proteins, evaluation of the fibrinolytic system and determination of von Willebrand Factor (vWF) and ADAMTS13. Laboratory data were compared with clinical data and outcomes. Data were analyzed using IBM SPSS Statistics for Mac, version 24. This study was approved by our institutional Ethics Committee and it was executed along with the international ethics recommendations for conducting research in humans following the latest revision of Declaration of Helsinki.
RESULTS
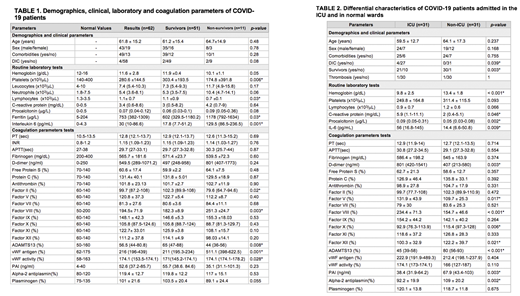
A total of 62 patients (31 ICU, 31 non-ICU) were analyzed. Mean age of the sample was 61.8 (SD 15.2) years and 69.4% of the patients were male. The coagulation parameters assessment demonstrated normal median PT, INR and APTT in our cohort and all coagulation factors were within normal range. Factor VIII showed an increasing trend (194.5±71.9) which could be interpreted as an acute phase reactant, and it was significantly higher in non-survivors (p=0.003). Similarly, we did not observe consumption of physiological inhibitor proteins and platelet counts were also within the normal limits, despite being slightly lower in non-survivor patients (p=0.006) (Table 1).
Von Willebrand Factor (vWF) was above the normal range (median 216%, IQR 196-439, normal range 62-175%) in our cohort and higher levels of vWF-antigen (p=0.001) and vWF-activity (p=0.02) were associated with poor prognosis. Likewise, a lower ADAMTS13 activity was observed in non-survivors (p=0.008). Regarding the fibrinolytic pathway, PAI levels were above the normal range (median 52.6ng/ml, IQR 37.2-85.7, normal range 4-40ng/ml), but we found no statistically significant differences based on survival. The remaining parameters of the fibrinolytic pathway (plasminogen and alpha-2 antiplasmin) were within normal range (Table 1).
ICU-patients had a poorer prognosis, with a higher rate of mortality (p=0.003). Likewise, they showed more elevated acute phase reactants (p<0.01) and higher levels of D-dimer (p=0.003) than patients hospitalized in general wards. ADAMTS13 activity was also significantly lower in this subgroup (p<0.001), as well as plasminogen levels (p=0.002) and PAI (p=0.003). Criteria of overt disseminated intravascular coagulation (DIC) as defined according to the ISTH DIC score, were met in 14.8% of ICU patients versus 0% of non-ICU patients (p=0.039) (Table 2).
CONCLUSIONS
COVID-19 infection is associated with coagulopathy that correlates with poor prognosis. However, coagulation factors, physiological inhibitory proteins and alpha-2-antplasmin levels were preserved in our study. Similarly, we did not observe platelets or fibrinogen consumption, which leads us to assume that COVID-19 coagulopathy is not a form of DIC. Increased vWF and decreased ADAMTS13 activity in our cohort could indicate that the underlying mechanism of this coagulopathy may reside in the endothelial cells and share a similar pathogenesis with thrombotic microangiopathy (TMA), as it has been recently suggested in some scientific reports.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal